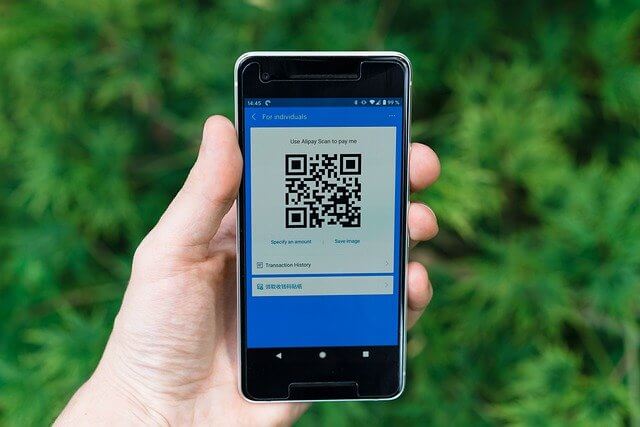
Simple but powerful QR Code Generator
Steps
1. Select QR Code type
2. Choose Foreground & Background Colors
3. Upload your logo
4. Paste your Content
5. Click on generate
6. TADA!! Download your QR Code in SVG or PNG format
An awesome tool to create personalised QR codes.
Ultimate QR Code Generator is a QR Code generator that allows us to create awesome personalised QR Code for free. It supports a variant of QR code types including URL, vCard, Email, Call, SMS, Whatsapp, WIFI, Paypal, and Bitcoin.
Know More About us